
Rare diseases can affect anyone at any time in their lives. There are more than 5000 rare diseases affecting up to almost 10% of the world’s population. However, only a small minority of these rare diseases have treatment (5).
Estimates vary as to how many rare diseases (RD) exist. This is, in part, because countries define these diseases differently:
- In European Union (EU) countries, any disease affecting fewer than 5 per 10,000 people is considered rare (European Commission Public Health Directorate) (5).
- The majority of patients suffer from diseases affecting one in 10,000 or less (European Medicines Agency) (1).
There is no single, widely accepted definition of a rare disease. The European Commission on Public Health defines rare diseases as those ‘diseases, including those of genetic origin, which are chronically debilitating or life-threatening. These diseases are so rare that special combined efforts are needed to combat them’ (5).
Rare diseases range from cystic fibrosis and haemophilia to Angelman syndrome, with an incidence of approximately 1 case per 15,000 population, and to Opitz trigonocephaly syndrome, which is extremely rare, with 1 case per million population (4).
The prevalence of RD will depend on the region being studied. For example, cystic fibrosis, which is a rare disease in Asia, but common in Europe, and also on the population studied, such as cancer in children.
GENETIC FACTORS
Most rare diseases are genetic in origin (80% of the total) (7,8) and therefore chronic: the result of infections, allergies and intoxications, or due to degenerative and proliferative causes; the effects of environmental exposure during pregnancy, or after birth, often in combination with genetic susceptibilities (8).
Among which we can highlight the following RD:
1.1 Arnold-Chiari syndrome (type 1):
It involves a descent of part of the cerebellum and sometimes almost the entire cerebellum through the greater occipital foramen (foramen magnum), thus compressing the brainstem. Familial aggregation studies (including concordant twins and monozygotic triplets) suggest a common genetic origin of Chiari malformation type 1 (11). A variant of Crouzon syndrome (most frequent subtype of craniosynostosis) is associated with Chiari syndrome type I and intracranial venous hypertension. Recent studies show that mutations in three of the four known FGFR genes are responsible for craniosynostosis (Crouzon, Apert, Pfeiffer and Jackon-Weiss)(6,16).
Studies by Meyers GA et al., 1995 and Mulliken JB et al., 1999(12), showed that Chiari type I and intracranial venous hypertension have a specific mutation in exon 10 of the FGFR3 locus (Ala391Glu).
However, Fujisawa et al., 2002(5), demonstrated that a mutation in exon IIIa, exon IIIc of the FGFR2 locus, Tyr281Cys, familial Crouzon syndrome was associated with Chiari I and Syringomyelia (2).
1.2 Lymphangioleiomyomatosis:
Typical disease in women of reproductive age, rare in men and children: proliferation of abnormal smooth muscle cells in the walls of the airways, blood vessels and lymphatic vessels of the lungs.
Mutations in the TSC1 gene or, more commonly, the TSC2 gene, cause LAM. The TSC1 and TSC2 genes provide the instructions for making the hamartin and tuberin proteins, respectively (14).
1.3 Hereditary spastic paraplegia
It is a neurodegenerative disorder characterised by progressive spasticity and hyperreflexia of the lower limbs. HSP can be inherited in an autosomal dominant, autosomal recessive or X-linked recessive manner, with multiple recessive and dominant forms occurring (3).
Only 11 autosomal and 2 X-linked genes have been identified, so the precise genetic basis for most HSP remains to be determined (13).
1.4 Friedreich’s ataxia
A neurodegenerative disease causing progressive deterioration of the cerebellum and dorsal spinal ganglia. This is an inherited disease with an autosomal recessive pattern of inheritance.
Mutations in the FXN gene (chromosome 9) and gene expression generate frataxin (appears to be important for normal mitochondrial function) (9).
1.5 Tarlov cyst
It is a disease of the presence of cysts filled with cerebrospinal fluid in the nerve roots, mainly located in the sacral area of the spine, causing painful radiculopathy (11).
It is a disease linked to a genetic defect, pathologies such as Ehler-Danlos, Marfan, Chiari, but, above all, spina bifida (polymorphisms in the MTHFR gene) and defects in collagen genes – which are expressed in the vast majority of chromosomes and have multiple expressions (15).
1.6 Prader Willi Syndrome
Usually complex in nature and with diverse causes and manifestations: Obesity, short stature, hypogonadism, cryptorchidism and learning disabilities following a stage of pre- and postnatal muscular hypotonia, in addition to mild to moderate intellectual disability (10).
The genetics of the disease are quite complex, and it is a classic example of what is known as genetic imprinting. It is associated with the loss of function of several genes on chromosome 15. The genes that provide instructions for generating nucleolar RNAs (snoRNAs). Several studies suggest that the loss of a particular group of snoRNA genes, known as the SNORD116 cluster, may play an important role in causing the signs and symptoms of Prader-Willi syndrome.
RARE DISEASES AND ASSISTED REPRODUCTIVE TECHNOLOGIES
Genetic diseases in general are serious, with no satisfactory prevention or treatment. Therefore, the only possible avenues of treatment are:
1. Prevention: genetic counselling to the population at risk and prenatal and/or pre-implantation diagnosis when possible.
2. Genetic counselling: communication process through which information is obtained on:
a. Risk of occurrence (occurrence)
b. Risk of recurrence (recurrence)
Preimplantation Genetic Diagnosis (PGD) is the technique that allows us to know the chromosomal or genetic endowment of the pre-embryo before it is transferred to the uterus and to avoid the interruption of the pregnancy. This technique can be carried out: 1. Preconceptionally or 2. Preimplantation
1. Preconceptionally. Chromosomal or gene analysis of the first polar corpuscle of the oocyte. Indications: elderly women, or women with structural abnormalities.
2. Preimplantation. Chromosomal analysis of one or more blastomeres of the pre-embryo/blastocyst. Indications: couples with a high risk of transmitting chromosomal or genetic abnormalities to their offspring.
Depending on the diagnosis, the molecular biology technique used for the genetic analysis of the embryo is as follows:
1. Polymerase chain reaction (PCR), allows for the detection of point mutations in single-gene diseases.
2. Fluorescent in situ hybridisation (FISH):
a) selection of embryos free of sex-linked diseases;
(b) study of numerical chromosomal abnormalities (haploidy, triploidy, nullisomies) and structural abnormalities (translocations and inversions).
Genetic diseases in general are serious, with no satisfactory prevention or treatment. Therefore, the only possible avenues of treatment are:
1. Prevention: genetic counselling to the population at risk and prenatal and/or pre-implantation diagnosis when possible.
2. Genetic counselling: communication process through which information is obtained on:
a. Risk of occurrence (occurrence)
b. Risk of recurrence (recurrence)
Preimplantation Genetic Diagnosis (PGD) is the technique that allows us to know the chromosomal or genetic endowment of the pre-embryo before it is transferred to the uterus and to avoid the interruption of the pregnancy. This technique can be carried out: 1. Preconceptionally or 2. Preimplantation
1. Preconceptionally. Chromosomal or gene analysis of the first polar corpuscle of the oocyte. Indications: elderly women, or women with structural abnormalities.
2. Preimplantation. Chromosomal analysis of one or more blastomeres of the pre-embryo/blastocyst. Indications: couples with a high risk of transmitting chromosomal or genetic abnormalities to their offspring.
Depending on the diagnosis, the molecular biology technique used for the genetic analysis of the embryo is as follows:
1. Polymerase chain reaction (PCR), allows for the detection of point mutations in single-gene diseases.
2. Fluorescent in situ hybridisation (FISH):
a) selection of embryos free of sex-linked diseases;
(b) study of numerical chromosomal abnormalities (haploidy, triploidy, nullisomies) and structural abnormalities (translocations and inversions).
It should be noted that the most prevalent rare genetic diseases are mostly single gene diseases: Arnold-Chiari syndrome, FGFR3 gene (chromosome 4) and FGFR2 gene (chromosome 9)… autosomal recessive inheritance; Friedreich’s Ataxia, FXN gene (chromosome 9)… autosomal recessive inheritance; Tarlov cyst, MTHFR gene… Autosomal dominant inheritance; Lymphangioleiomyomatosis, TSC1 gene (chromosome 9 (9q34)) and TSC2 gene (chromosome 16 (16p13))… sex-linked inheritance; Hereditary spastic paraplegia, sex-linked inheritance; other gene/chromosomal abnormalities: Prader Willi syndrome, deletion of several genes (chromosome 15)… genomic imprinting(16).
Chromosomal abnormalities relate to embryonic morphology. There are two types of chromosomal abnormalities in the embryo:
1. Those occurring during meiosis: aneuploidy.
2. Those occurring after meiosis: mosaicism, polyploidy and haploidy.
It is necessary to take into account that morphology is a valid tool for only some chromosomal abnormalities. Therefore, the best selection method will be pre-implantation genetic diagnosis and embryo morphology. According to Rubio et al, 2007(18), the rate of development to blastocyst is influenced by the possible chromosomal alterations, with the following being observed: Euploids…66%, Trisomies…37%, Monosomies…9%, Polyploids…21%, Haploids…0%, Diploid mosaics…13%.
The algorithm for embryo selection in patients with RD susceptible to Preimplantation Genetic Diagnosis could be defined as follows:
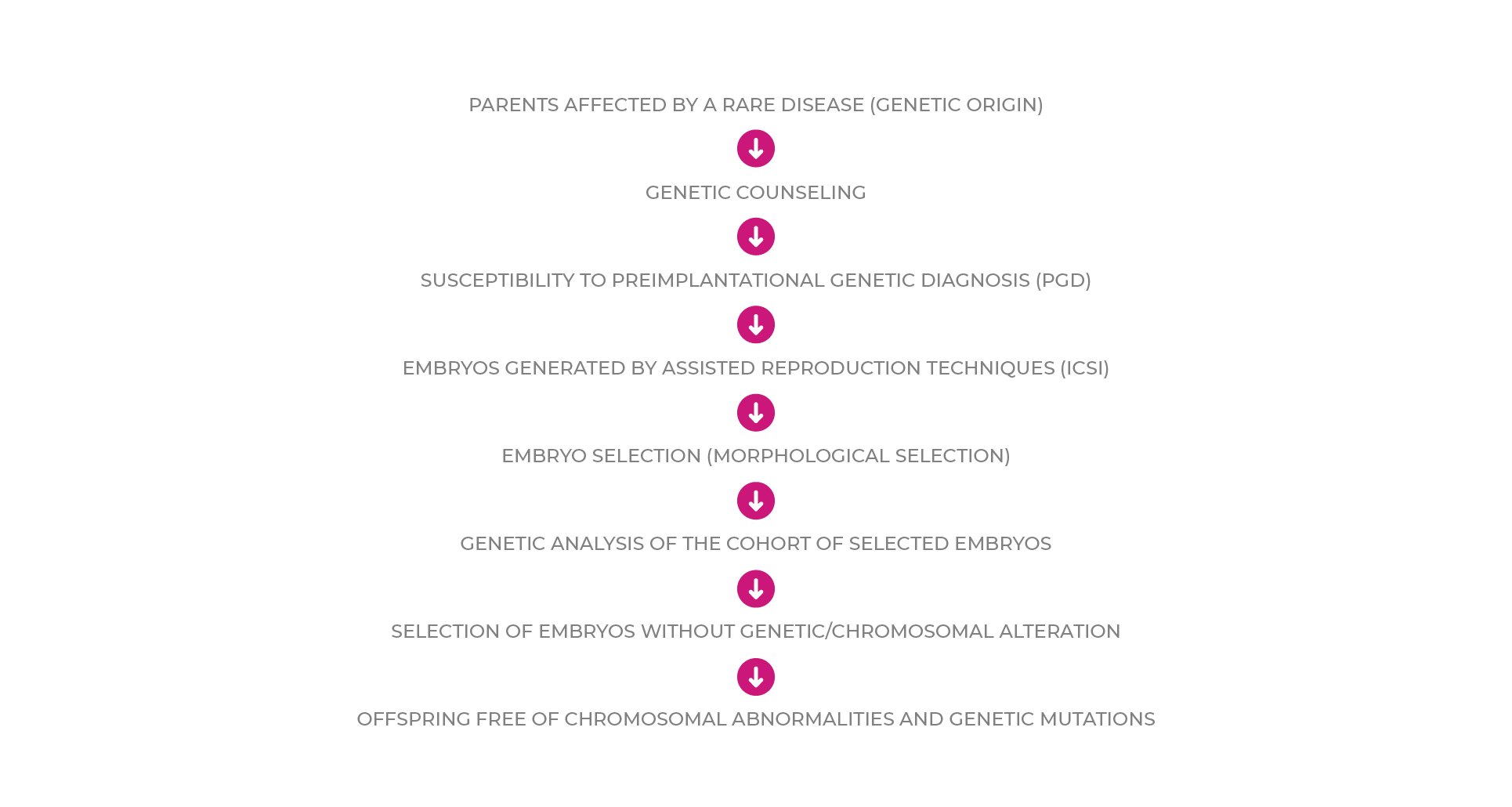
CONCLUSIONS
1. Prolonged culture allows embryo selection (pre-embryos with high implantation power) but not free of chromosomal abnormalities.
2. The combination of embryo selection and Preimplantation Genetic Diagnosis is very useful in patients with RD.
3. PGD allows obtaining pre-embryos free of chromosomal abnormalities and genetic mutations.




